Coq10 Vitamin C
- Research
- Open Access
- Published:
Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors
Nutrition & Metabolism volume 7, Article number:55 (2010) Cite this article
-
39k Accesses
-
59 Citations
-
15 Altmetric
-
Metrics details
Abstract
Background
Antioxidant supplementations have the potential to alleviate the atherosclerotic damage caused by excessive production of reactive oxygen species (ROS). The present study evaluated the effects of prolonged antioxidant treatment on arterial elasticity, inflammatory and metabolic measures in patients with multiple cardiovascular risk factors.
Methods
Study participants were randomly assigned to two groups. Group 1 received oral supplementation with 2 capsules per day of Mid Life Guard, SupHerb, Israel. In each capsule vitamin C (500 mg) vitamin E (200 iu), co-enzyme Q10 (60 mg) and selenium (100 mcg), Group 2 received matching placebo(SupHerb) for 6 months. Patients were evaluated for lipid profile, HbA1C, insulin, C-peptide, hs-CRP, endothelin, aldosterone, plasma renin activity and Homeostasis model assessment-insulin resistance (HOMA-IR). Arterial elasticity was evaluated using pulse wave contour analysis (HDI CR 2000, Eagan, Minnesota).
Results
Antioxidant-treated patients exhibited significant increases in large arterial elasticity index (LAEI) as well as small arterial elasticity index (SAEI). A significant decline HbA1C and a significant increase in HDL-cholesterol were also observed. In the placebo group, significant changes in LAEI, SAEI or metabolic measures were not observed.
Conclusions
Antioxidant supplementation significantly increased large and small artery elasticity in patients with multiple cardiovascular risk factors. This beneficial vascular effect was associated with an improvement in glucose and lipid metabolism as well as decrease in blood pressure.
Background
Oxidative stress has been considered as a potential pathogenic mechanism for initiation and progression of atherosclerotic disease [1]. Excessive production of reactive oxygen species, a mediator of the oxidative stress cascade, leads to release of inflammatory cytokines, oxidation of LDL and prothrombotic state, and finally results in endothelial dysfunction and atherosclerotic vascular lesions [2–4]. Adequate dietary, enzymatic and nonenzymatic antioxidant supplementation may be effective in lowering oxidative stress. Although most trials with antioxidants in experimental models of atherosclerosis have demonstrated that this treatment is able to retard the progression of atherosclerosis, the results of clinical trials are equivocal [5]. Differences in the definition criteria of patients who are potential candidates for antioxidant treatment, type and dosage of antioxidant supplementation, as well as outcome measures may explain this variability. Since oxidative stress is activated by many cardiovascular risks factors such as hypertension, hyperglycemia, dyslipidemia and smoking, patients with multiple cardiovascular risk factors could obtain beneficial effect from antioxidant treatment [6–9]. Additionally, combinations of dietary antioxidants (vitamin C and vitamin E) with carrier in mitochondrial oxidative phosphorylation (coenzyme Q10) and trace elements essential for adequate function of many antioxidant enzymes (selenium) may underlie the synergism between them and amplify the positive antioxidant effect. The present study was designed to determine the effect of antioxidant supplementation with vitamin C, vitamin E, coenzyme Q10 and selenium on arterial compliance, inflammatory and metabolic parameters in patients with multiple cardiovascular risk factors.
Methods
In a randomized, placebo-controlled study 70 patients with at least two cardiovascular risk factors were recruited from the hypertension outpatient clinic at E.Wolfson Medical Center for study participation. Screening procedures included physical examination, complete blood chemistry; complete blood count, urinalysis and electrocardiogram.
Cardiovascular risk factors were defined using the National Cholesterol Education Program risk factors categories: hypertension (systolic blood pressure > = 140 mm Hg and/or diastolic BP > = 90 mm Hg and/or taking antihypertensive medication); diabetes (fasting plasma glucose level > = 126 mg/dl on at least two blood samples and/or taking glucose lowering agents, hypertriglyceridemia (> = 1.7 mmol/l); low HDL cholesterol level (< 1.03 mmol/l in men and < 1.3 mmol/l in women); or current cigarette smoking.
Patients with a history of unstable angina, MI, CVA or major surgery within the six months preceding entrance to the study were excluded. Patients with unbalanced endocrine disease or any disease that might affect absorption of medications were excluded, as were patients with plasma creatinine > 2 mg/dl, elevation of liver enzymes to more that twice the upper normal limit, and electrolyte abnormalities. Patients included in the study were stabilized on their previous medical treatment in the outpatient clinic for up to three months, and an effort was made not to change treatment during the study. All concomitant medications were kept stable to prevent possible effects on the study parameters.
The study was approved by the Institutional Review Board and the patients signed a full informed consent.
Study participants were randomly assigned to two groups. Group 1 received oral supplementation with 2 capsules per day of Mid Life Guard, SupHerb, Israel. In each capsule vitamin C (500 mg) vitamin E (200 iu), co-enzyme Q10 (60 mg) and selenium (100 mcg), Group 2 received matching placebo(SupHerb) for 6 months.
Biochemical parameters
Blood sampling for full chemistry and metabolic parameters, including fasting glucose, lipid profile, HbA1C, hs-CRP, homocysteine, endothelin, aldosterone, plasma renin activity was performed at baseline and at the end of the study.
Arterial Elasticity Measurements
Arterial compliance measures were performed after an overnight fast and before blood sampling. Measures were performed between 8 and 10 AM, in a quiet, temperature -controlled laboratory. With the subject in a supine position, radial arterial waveforms were recorded for 30 sec. The pressure transducer amplifier system was connected to a specially designed device (Model CR-2000, Hypertension Diagnostics Inc. Eagan, MN). The passive transient response of the arterial vasculature to the initial loading conditions was determined by analyzing the diastolic portion of the pressure pulse-wave form. This technique, which has been validated for its reproducibility and used extensively [10–12], was performed with a simple noninvasive radial pulse wave recording and computer analysis of the diastolic decay. This provides separate assessment of the large artery or capacitive compliance (C1) and small artery reflective or oscillatory compliance (C2). Cardiac output and stroke volume were computed from the average waveforms. Systemic vascular resistance (SVR) is calculated as mean arterial pressure (MAP) divided by cardiac output (CO). Arterial elasticity was determined at the baseline visit and at the 3- and 6 -month on-treatment visits.
Sample size
The primary endpoint in the present study was the paired difference in small artery elasticity (SAEI) within each group. With a sample size of n = 33 in each group, the present study was designed to have not less than 80% power to detect a true, within group difference of at least 1.2 ± 2 mL/mm Hg × 100 in SAEI in each of the two treatment groups assuming a two-tailed alpha of 0.025 (preserving to overall study alpha at 0.05).
Statistical analysis
Analysis of data was carried out using SPSS 10.0 statistical analysis software (SPSS Inc., Chicago, IL, USA, 1999). For continuous variables, such arterial compliance parameters and biochemistry data, descriptive statistics were calculated and reported as mean ± standard deviation. Normalcy of distribution of continuous variables was assessed using the Kolmogorov-Smirnov test (cut off at p = 0.01). Categorical variables such as sex and concomitant illnesses were described using frequency distributions and are presented as frequency (%). The t-test for independent samples was used to compare continuous variables by treatment group. Categorical variables were compared by treatment group using the chi square test (exact as needed). General linear modeling was used to compare post-treatment continuous variables that differed by treatment group at baseline, including treatment group as the fixed factor and the baseline value of the modeled variable as a covariate. All tests are two-sided and considered significant at p < 0.05.
Results
Demographic and clinical characteristics of the 70 patients with multiple cardiovascular risk factors are presented in Table 1. Group 1 included 36 patients who received oral daily supplementation with vitamin C, vitamin E, coenzyme Q10 and selenium. Group 2 included 34 patients who received placebo. As can be seen, both groups were similar with respect to age, sex, BMI, presence of cardiovascular risk factors, baseline blood pressure level and arterial elasticity parameters. Concomitant medications were similarly distributed in both groups at the start and end of the study.
Full size table
Changes in hemodynamic and arterial elasticity parameters in patients treated with antioxidants
Table 2 shows six month follow-up of hemodynamic and arterial elasticity parameters in patients received antioxidants. Systolic blood pressure (SBP) decreased significantly from 145.2 +/- 25.4 at baseline to 136.1 +/- 22.3 mmHg after 6 months of treatment (p < 0.001). Diastolic blood pressure (DBP) decreased significantly during the treatment period from 78.4 +/- 11.7 to 75.0 +/- 12.3 mmHg (p < 0.034). Heart rate did not change during the study.
Full size table
LAEI increased from 11.0 +/- 4.4 to 12.7 +/- 4.7 ml/mm Hg × 100 after 6 months of treatment (p < 0.006) (Figure 1). SAEI increased significantly during the study from 3.3 +/- 1.9 to 4.7 +/- 2.7 ml/mm Hg × 100 (p < 0.0001) (Figure 2). Although SVR decreased by 11% during the treatment period, this decrease did not reach statistical significance (p = 0.102) (Table 2).
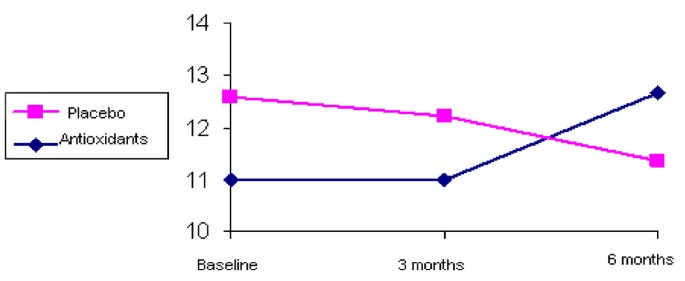
LAEI by groups during 6-month follow-up.
Full size image
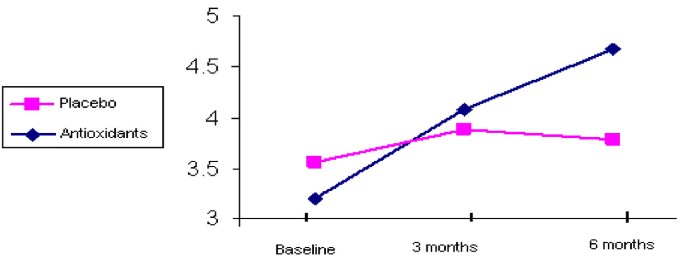
SAEI by groups during 6-month follow-up.
Full size image
Changes in hemodynamic and arterial elasticity parameters in the control group
As shown in Table 2, systolic blood pressure as well as diastolic blood pressure did not change significantly during the study (p = 0.257 and p = 0.493, respectively).
Neither LAEI nor SAEI improved significantly during the treatment period. LAEI was 12.9 +/- 6.2 ml/mmHg × 100 at baseline and 11.3 +/- 4.3 ml/mmHg × 100 after 6 months of follow-up (p = 0.151) (Figure 1). SAEI was 3.6 +/- 1.5 at baseline and 3.7 +/- 1.9 ml/mmHg × 100 at the end of the study (p = 0.732) (Figure 2). SVR tended to increase during the study from 1594.7 +/- 351.3 to 1638.7 +/- 311.3 dyne × sec × cm, however this increase did not reach statistical significance (p = 0.533) (Table 2).
Changes in metabolic and inflammatory parameters during 6-month treatment period
As shown in Table 2, levels of HbA1C decreased significantly from 7.08 ± 1.69% to 6.33 ± 2.3% (p = 0.022) in patients received antioxidants. Additionally, significant increase in HDL-cholesterol (p = 0.022) and marginal declines in triglycerides (p = 0.058) as well as total cholesterol levels (p = 0.074) were observed in antioxidant treated patients.
Metabolic parameters including HbA1C, triglycerides, total cholesterol, HDL-cholesterol and CRP did not change in placebo group during the study.
Discussion
The present randomized, placebo controlled study demonstrates that antioxidant supplementation with vitamin C, vitamin E, coenzyme Q10 and selenium significantly increased large and small artery elasticity in patients with multiple cardiovascular risk factors. This beneficial vascular effect was associated with an improvement in glucose and lipid metabolism as well as significant decrease in blood pressure.
Assessment of arterial function and structure can serve as a surrogate endpoint for prediction of morbid events and for estimation of success of treatment. Pulse wave contour analysis using the modified Windkessel model is one of several noninvasive methods for estimation of arterial properties. Numerous studies performed with the HDI CR-2000 equipment have shown good correlation to age, cardiovascular risk factors and markers of disease [13]. Therapeutic interventions with medications like statins, angiotensin II receptor blocking agents as well as weight loss, have also shown improvement in LAEI and SAEI which may suggest lowering of cardiovascular risk. Nevertheless, no major prospective study associating arterial elasticity with cardiovascular events has been performed. Although PWA using the modified Windkessel model has some limitations, this method provides complementary information about vascular health.
The favorable vascular effect of antioxidants has been observed in vitro and in animal models of atherosclerosis [14–16]. However, data on long-term vascular impact of antioxidant supplementation in humans are limited and controversial. The findings of the present study concur with those of previous study that has shown substantial reduction in the progression of common carotid atherosclerosis during three year treatment with combined supplementation of both vitamin E and vitamin C [17]. Additionally, the beneficial effect of antioxidant supplementation on LDL oxidation and endothelial flow has been demonstrated [18, 19]. Moreover several prospective randomized controlled clinical trials such as Cambridge Heart Antioxidant Study, Secondary Prevention with Antioxidants of Cardiovascular Disease in End-stage Renal Disease study and Cholesterol Lowering Atherosclerosis Study reported that the administration of antioxidants reduced the risk of cardiovascular disease [20–22]. Nevertheless, subsequent large interventional studies do not support a benefit from antioxidant supplementation [23, 24]. These clinical trials have demonstrated that vitamin E alone or in combination has no effect on the risk of death or prevention of cardiovascular disease. Moreover, a dose-response meta-analysis has shown that high-dosage vitamin E supplementation was associated with a small but statistically significant increased risk for mortality [25]. The lack of benefit seen in these clinical trials does not disprove the central role of oxidative stress in atherosclerosis and justify investigating the overall clinical impact of antioxidant treatment.
Although the anti-atherogenic effect of antioxidants has been assessed in several experimental studies, the mechanisms by which these agents inhibit atherosclerosis remain to be clarified. Combined supplementation of vitamin E and C have been shown to inhibit DNA oxidation by H2O2 in human lymphocytes, to enhance endogenous plasma and tissue antioxidant defenses and restore endothelium-dependent vasoactivity [18, 26, 27]. Coenzyme Q10 which plays an essential role as an electron carrier in mitochondrial oxidative phosphorylation, improves endothelial dysfunction in diabetic patients [28]. Finally, selenium as a determinant of antioxidative glutathione peroxidase 1 expression and activity, provides significant protection of the coronary artery endothelium against damage by oxidative stress [29].
The findings of the present study concur with those of previous studies that have shown substantial reduction in blood pressure and improvement in long-term glycaemic control with oral CoQ supplementation, reduction in plasma glucose and insulin resistance with high doses of vitamin E supplementation and significant reduction in blood pressure levels with vitamin E as well as vitamin C in hypertensive patients [30–33]. Nevertheless, which particular antioxidant or combination of antioxidants is responsible for the favorable metabolic effect in the present study remains uncertain. Additionally, we cannot exclude the possibility that specific antioxidant combination which was used in the present study, has a contributory effect of on blood pressure, glucose and lipid homeostasis as well as on improvement of vascular elasticity.
In the present study, we did not observe significant changes in humoral factors such as homocystein, endothelin, aldosterone and renin in subjects received antioxidant supplementation. Levels of urine cathecholamines also did not change during the treatment period. These findings emphasize a previously published data which have shown that patophysiologic mechanism of antioxidants action is independent of the changes in plasma concentration of blood pressure modulators, such as renin, aldosterone, endothelin [34], although the precise mechanism for antioxidant action on the vasculature remains to be elucidated.
Our study has several limitations. First, the present study contains relatively small number of participants and larger studies are required to establish the beneficial vascular effect of antioxidant supplementation. Second, we did not measure plasma levels of the antioxidants which would have added the information regarding treatment compliance and would have elucidated the pathophysiology for vascular action of antioxidants. Furthermore, since the present study has focused on patients with multiple cardiovascular risk factors, the application of our findings to other patient populations remains uncertain.
Conclusion
We have demonstrated that combined antioxidant supplementation with vitamin C, vitamin E, coenzyme Q10 and selenium has beneficial effect on glucose and lipid metabolism, blood pressure and arterial compliance in patients with multiple cardiovascular risk factors. The findings of the present study justify investigating the overall clinical impact of antioxidant treatment in this population.
Abbreviations
- CI:
-
confidence interval
- NSAIDs:
-
non-steroidal anti-inflammatory drugs
- SD:
-
standard deviation.
References
- 1.
Stocker R, Keaney JF: Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004, 84: 1381-1478. 10.1152/physrev.00047.2003.
CAS Article Google Scholar
- 2.
Schleicher E, Friess U: Oxidative stress, AGE, and atherosclerosis. Kidney International. 2007, 72: S17-S26. 10.1038/sj.ki.5002382.
Article Google Scholar
- 3.
Griendling KK, FitzGerald GA: Oxidative stress and cardiovascular injury: Part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003, 108: 1912-1916. 10.1161/01.CIR.0000093660.86242.BB.
Article Google Scholar
- 4.
Kunsch C, Medford RM: Oxidative stress as a regulator of gene expression in the vasculature. Circ Res. 1999, 85: 753-766.
CAS Article Google Scholar
- 5.
Madamanchi NR, Hakim ZS, Runge MS: Oxidative stress in atherogenesis and arterial thrombosis: the disconnect between cellular studies and clinical outcomes. J Thromb Haemost. 2005, 3: 254-267. 10.1111/j.1538-7836.2004.01085.x.
CAS Article Google Scholar
- 6.
Maxwell SR, Thomason H, Sandler D, LeGuen C, Baxter MA, Thorpe GH, Jones AF, Barnett AH: Poor glycaemic control is associated with reduced serum free radical scavenging (antioxidant) activity in non-insulin-dependent diabetes mellitus. Ann Clin Biochem. 1997, 34: 638-644.
Article Google Scholar
- 7.
Sanguigni V, Pignatelli P, Caccese D, Pulcinelli FM, Lenti L, Magnaterra R, Martini F, Lauro R, Violi F: Increased superoxide anion production by platelets in hypercholesterolemic patients. Thromb Haemost. 2002, 87: 796-801.
CAS Google Scholar
- 8.
Beswick RA, Dorrance AM, Leite R, Webb RC: NADH/NADPH oxidase and enhanced superoxide production in the mineralocorticoid hypertensive rat. Hypertension. 2001, 38: 1107-11. 10.1161/hy1101.093423.
CAS Article Google Scholar
- 9.
Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, Strauss WE, Oates JA, Roberts LJ: Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers. Smoking as a cause of oxidative damage. N Engl J Med. 1995, 332: 1198-203. 10.1056/NEJM199505043321804.
CAS Article Google Scholar
- 10.
Zieman SJ, Melenovsky V, Kass DA: Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005, 25: 932-943. 10.1161/01.ATV.0000160548.78317.29.
CAS Article Google Scholar
- 11.
Giannattasio C, Mancia G: Arterial distensibility in humans. Modulating mechanisms, alterations in diseases and effects of treatment. J Hypertens. 2002, 20: 1889-1899. 10.1097/00004872-200210000-00001.
CAS Article Google Scholar
- 12.
Cohn JN, Finkelstein S, McVeigh G, Morgan D, LeMay I, Robinson J, Mock J: Non- invasive pulse wave analysis for the detection of arterial vascular disease. Hypertension. 1995, 26: 503-508.
CAS Article Google Scholar
- 13.
Zimlichman R, Shargorodsky M, Boaz M, Duprez D, Rahn D, Rizzoni D, Payeras AC, Hamm C, McVeigh G: Determination of arterial compliance using blood pressure waveform analysis with the CR-2000 system: Reliability, repeatability, and establishment of normal values for healthy European population. Am J Hypertens. 2005, 18 (1): 65-71. 10.1016/j.amjhyper.2004.08.013.
Article Google Scholar
- 14.
Hsich E, Segal BH, Pagano PJ, Rey FE, Paigen B, Deleonardis J, Hoyt RF, Holland SM, Finkel T: Vascular effects following homozygous disruption of p47(phox): An essential component of NADPH oxidase. Circulation. 2000, 101: 1234-1236.
CAS Article Google Scholar
- 15.
Chen X, Touyz RM, Bae Park J, Schiffrin EL: Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001, 38: 606-611. 10.1161/hy09t1.094005.
CAS Article Google Scholar
- 16.
Wu BJ, Kathir K, Witting PK, Beck K, Choy K, Li C, Croft KD, Mori TA, Tanous D, Adams MR, Lau AK, Stocker R: Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med. 2006, 203: 1117-1127. 10.1084/jem.20052321.
CAS Article Google Scholar
- 17.
Salonen JT, Nyyssönen K, Salonen R, Lakka HM, Kaikkonen J, Porkkala-Sarataho E, Voutilainen S, Lakka TA, Rissanen T, Leskinen L, Tuomainen TP, Valkonen VP, Ristonmaa U, Poulsen HE: Antioxidant Supplementation in Atherosclerosis Prevention Study (ASAP): a randomized trial of the effect of vitamins E and C on 3-year progression of carotid atherosclerosis. J Intern Med. 2000, 248: 377-386. 10.1046/j.1365-2796.2000.00752.x.
CAS Article Google Scholar
- 18.
Plotnick GD, Corretti MC, Vogel RA: Effect of antioxidant vitamins on the transient impairment of endothelium-dependent brachial artery vasoactivity following a single high-fat meal. JAMA. 1997, 278: 1682-1686. 10.1001/jama.278.20.1682.
CAS Article Google Scholar
- 19.
Devaraj S, Jialal I: Low-density lipoprotein postsecretory modification, monocyte function, and circulating adhesion molecules in type 2 diabetic patients with and without macrovascular complications: the effect of α-tocopherol supplementation. Circulation. 2000, 102: 191-196.
CAS Article Google Scholar
- 20.
Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ: Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet. 1996, 347: 781-786. 10.1016/S0140-6736(96)90866-1.
CAS Article Google Scholar
- 21.
Boaz M, Smetana S, Weinstein T, Matas Z, Gafter U, Iaina A, Knecht A, Weissgarten Y, Brunner D, Fainaru M, Green MS: Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet. 2000, 356: 1213-1218. 10.1016/S0140-6736(00)02783-5.
CAS Article Google Scholar
- 22.
Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, Dowdy AA, Marino EK, Bolson EL, Alaupovic P, Frohlich J, Albers JJ: Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001, 345: 1583-1592. 10.1056/NEJMoa011090.
CAS Article Google Scholar
- 23.
Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P: Vitamin E supplementation and cardiovascular events in high-risk patients. Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000, 342: 154-160. 10.1056/NEJM200001203420302.
CAS Article Google Scholar
- 24.
Collaborative Group of the Primary Prevention Project: Low-dose aspirin and vitamin E in people at cardiovascular risk: a randomized trial in general practice. Lancet. 2001, 357: 89-95. 10.1016/S0140-6736(00)03539-X.
Article Google Scholar
- 25.
Miller ER, Pastor-Barriuso R, Dalal D, Riemersma RA, Appel LJ, Guallar E: Meta-analysis: high-dosage vitamin E supplementation may increase all-cause mortality. Ann Intern Med. 2005, 142: 37-46.
CAS Article Google Scholar
- 26.
Brennan LA, Morris GM, Wasson GR, Hannigan BM, Barnett YA: The effect of vitamin C or vitamin E supplementation on basal and H2O2-induced DNA damage in human lymphocytes. Br J Nutr. 2000, 84: 195-202. 10.1079/096582197388680.
CAS Article Google Scholar
- 27.
Dusinská M, Kazimírová A, Barancoková M, Beno M, Smolková B, Horská A, Raslová K, Wsólová L, Collins AR: Nutritional supplementation with antioxidants decreases chromosomal damage in humans. Mutagenesis. 2003, 18: 371-376. 10.1093/mutage/geg002.
Article Google Scholar
- 28.
Watts GF, Playford DA, Croft KD, Ward NC, Mori TA, Burke V: Coenzyme Q10 improves endothelial dysfunction of the brachial artery in type II diabetes mellitus. Diabetologia. 2002, 45: 420-426. 10.1007/s00125-001-0760-y.
CAS Article Google Scholar
- 29.
Miller S, Walker SW, Arthur JR, Nicol F, Pickard K, Lewin MH, Howie AF, Beckett GJ: Selenite protects human endothelial cells from oxidative damage and induces thioredoxin reductase. Clin Sci (Lond). 2001, 100 (5): 543-50. 10.1042/CS20000299.
CAS Article Google Scholar
- 30.
Hodgson JM, Watts GF, Playford DA, Burke V, Croft KD: Coenzyme Q10 improves blood pressure and glycaemic control: a controlled trial in subjects with type 2 diabetes. European Journal of Clinical Nutrition. 2002, 56: 1137-1142. 10.1038/sj.ejcn.1601464.
CAS Article Google Scholar
- 31.
Manning PJ, Sutherland WH, Walker RJ, Williams SM, De Jong SA, Ryalls AR, Berry EA: Effect of high-dose vitamin E on insulin resistance and associated parameters in overweight subjects. Diabetes Care. 2004, 27: 2166-2171. 10.2337/diacare.27.9.2166.
CAS Article Google Scholar
- 32.
Boshtam M, Rafiei M, Sadeghi K, Sarraf-Zadegan N: Vitamin E can reduce blood pressure in mild hypertensives. Int J Vitam Nutr Res. 2002, 72: 309-314. 10.1024/0300-9831.72.5.309.
CAS Article Google Scholar
- 33.
Duffy SJ, Gokce N, Holbrook M, Huang A, Frei B, Keaney JF, Vita JA: Treatment of hypertension with ascorbic acid. Lancet. 1999, 354: 2048-2049. 10.1016/S0140-6736(99)04410-4.
CAS Article Google Scholar
- 34.
Rodrigo R, Prat H, Passalacqua W, Araya J, Bächler JP: Decrease in oxidative stress through supplementation of vitamins C and E is associated with a reduction in blood pressure in patients with essential hypertension. Clin Sci (Lond). 2008, 114 (10): 625-34. 10.1042/CS20070343.
CAS Article Google Scholar
Download references
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MS, OD, ZM and RZ contributed to the study conception and design. MS, OD and RZ were responsible for the data acquisition. RZ and OD performed the analysis and interpretation of data. ZM carried out the immunoassays. MS were responsible for review the existing literature and for writing the first draft of the paper. All authors performed a critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Authors' original submitted files for images
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and Permissions
About this article
Cite this article
Shargorodsky, M., Debby, O., Matas, Z. et al. Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr Metab (Lond) 7, 55 (2010). https://doi.org/10.1186/1743-7075-7-55
Download citation
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/1743-7075-7-55
Keywords
- Selenium
- Arterial Compliance
- Antioxidant Supplementation
- Antioxidant Treatment
- Arterial Elasticity
Source: https://nutritionandmetabolism.biomedcentral.com/articles/10.1186/1743-7075-7-55

0 Komentar